Biomedical applications of functionalized carbon and 2D nanomaterials
We exploited different types of functionalized CNTs for targeting, drug delivery, and gene silencing. In particular, we explored various strategies for the conjugation of antibodies onto CNTs, through amidation or thiol-ene ligation, to target cancer cells (1). We demonstrated that CNTs conjugated to the antibody Cetuximab were able to target the epidermal growth factor receptor, which is overexpressed by several cancer cells (1). We also functionalized CNTs with a mitochondrial targeting peptide and observed the localisation of the nanotubes into these organelles (2). Targeting mitochondria might open the way to develop alternative systems to tackle diseases related to genetic mutations in mitochondrial DNA, by delivering therapeutic oligonucleotides. We also reported the controlled covalent derivatization of triple-functionalized CNTs with the anticancer drug gemcitabine, folic acid as a targeting ligand of cancer cells, and fluorescein for imaging (3). The anticancer activity of gemcitabine was maintained after covalent grafting onto the CNTs. Nevertheless, the functionalized nanotubes were internalised into both folate-positive and negative cells, suggesting the passive diffusion of CNTs. We studied the in vivo biodistribution of platinum-based drugs encapsulated into multi-walled CNTs (4) and we showed that the drug release kinetics depends on the nanotube diameter (5). Indeed, smaller CNT diameter allows slower drug release inside tumor cells compared to CNTs with a larger diameter. We also designed cationic CNTs as efficient siRNA vectors for lung cancer xenograft eradication in comparison to liposomes (6). Furthermore, we showed the functional motor recovery from brain ischemic injury by CNT-mediated siRNA silencing (7). In another study we decorated the surface of CNTs with iron oxide nanoparticles (NPs) via different strategies to impart magnetic properties to the nanotubes (8). The NP/CNT hybrids were exploited for cell labeling, magnetic resonance imaging cell tracking, and magnetic manipulations. We investigated the use of different types of carbon nanomaterials for photothermal ablation of tumor cells, for instance multi-walled CNTs filled with iron oxide NPs (9) and carbon nanohorns coated with magnetite NPs (10). Combination of photothermal therapy (PTT) with photodynamic therapy (PDT) was performed using CNTs conjugated to a photosensitizer (11) (Figure 1) or dye-functionalized carbon nanohorns (12).
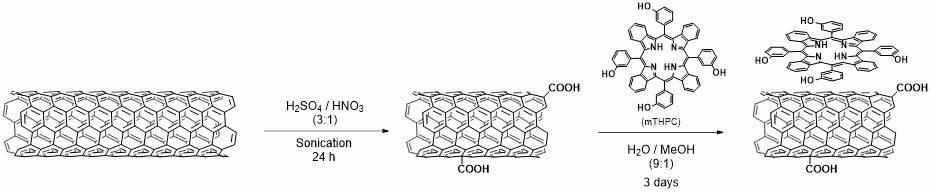
Due to their unique properties, CNTs have attracted a great attention for applications as components of advanced biomaterials for regenerative medicine. In this context, we evaluated the use of CNTs functionalized with a fibroblast growth factor (FGF) as scaffolds for bone formation in vitro and in vivo (13) The presence of FGF enhanced the proliferation of rat bone marrow stromal cells. Finally, we did pioneering studies on the use of amino-functionalized CNTs as ultrasound contrast agents for echography (14). We carried out a thorough investigation to assess the echogenic properties of the nanotubes, giving support for their future applications as theranostic nanoparticles, combining diagnostic and therapeutic modalities.
The graphene-family nanomaterials have also captured the attention of an increasing number of scientists for applications in different fields. In nanomedicine graphene holds great interest in drug delivery, biosensing and tissue engineering (15, 16). Graphene oxide (GO), the oxidized form of graphene, shows high potential in this field. Indeed, the polar oxygenated functional groups on its surface make it highly hydrophilic, leading to a good dispersibility in water. We developed a novel platform to complex small interference RNA (siRNA) molecules using GO samples with various percentage of oxygen (17). GO was covalently functionalized with triethyleneglycole-diamine and with low molecular weight polyethyleneimine (PEI, 800 Da) via the epoxy ring opening reaction. By gel electrophoresis, we were able to correlate the GO complexation ability with the graphene surface chemistry. GO functionalized with PEI showed a high complexing capacity for siRNA, proving to be a promising candidate for gene silencing
We also investigated the interactions between GO and siRNA molecules (18). We focused on how the GO size, oxygenated groups present on the surface and chemical functionalisation affect the double helix siRNA structure. We observed that the siRNA secondary structure was clearly altered by the interaction with GO flakes. Moreover, we were able to correlate the double strand damage with the size and the oxygenated groups present on GO. Finally, we demonstrated that GO functionalized with PEI was able to protect siRNA from structural modifications.
Our expertise on the multifunctionalisation of CNTs and GO allows the design of multimodal conjugates for the treatment of different diseases. We wish to exploit the intrinsic properties of CNTs to absorb near-infrared light and convert it into heat, with the objective to selectively destroy the targeted diseased cells by photothermal therapy, in combination with other therapies for the treatment of autoimmune diseases and cancer.
- a) Venturelli, E. et al. (2011) Small 7, 2179-2187. b) Spinato, C. et al. (2016) Nanoscale 8, 12626-12638.
- Battigelli, A. et al. (2013) Nanoscale 5, 9110-9117.
- Ménard-Moyon, C. et al. (2015) Chem. Eur. J. 21, 144886-14482.
- Li, J. et al. (2014) Nanomedicine NMB 10, 1465-1475.
- Muzi, L. et al. (2015) Nanoscale 7, 5383-5394.
- Guo, C. et al. (2015) Bioconjug. Chem. 26, 1370-1379.
- Al-Jamal, K. T. et al. (2011) Proc. Natl Acad. Sci. U. S. A. 108, 10952-10957.
- Lamanna, G., et al. (2013) Nanoscale 5, 4412-4421.
- Liu, X. et al. (2014) ACS Nano 8, 11290-11304.
- Chechetka, S. A. et al. (2015) Chem. Asian J. 10, 160-165.
- Marangon, I. et al. (2016) Carbon 97, 110-123.
- Miyako, E. et al. (2014) Angew. Chem. Int. Ed. Engl. 53, 13121-13125.
- Hirata, E. et al. (2013) Nanotechnology 24, 435101.
- Delogu, L. G. et al. (2012) Proc. Natl Acad. Sci. U. S. A. 109, 16612-16617.
- Kurapati, R. et al. (2016) Adv. Mater. 28, 6052-6074.
- Reina, G. et al. (2017) Chem. Soc. Rev. 46, 4400-4416.
- Chau, N. D. Q. (2017) Carbon 122, 643-652.
- Reina, G. et al. (2018) Nanoscale 10, 5965-5974.
 Intranet
Intranet Access
Access Contact
Contact
