Back
Our analyses

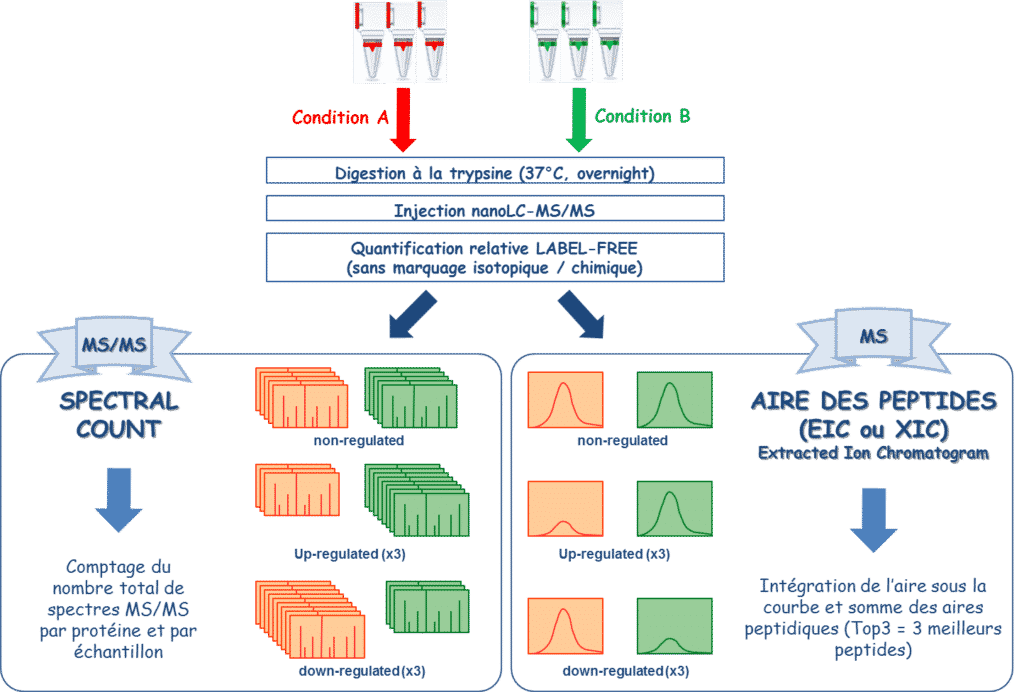
Proteomics is mainly the analysis of the proteins constituting a complex protein mixture. In “bottom up” mass spectrometry, it’s the peptides resulting from an enzymatic digestion of proteins which are analyzed. In recent years, the trend has been reversed: digestion using SDS-PAGE gels has given way to digestions of proteins directly in solution.

Our facility specialized in affinity co-immunoprecipitation strategies or co-IPs.
 Intranet
Intranet Access
Access Contact
Contact