Targeting HIV-1 Dimerization Initiation Site with small molecules
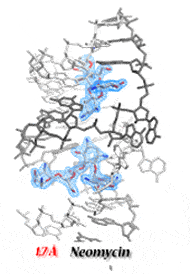
HIV-1 Dimerization Initiation Site (DIS) is a conserved hairpin in the 5’ UTR of the genomic RNA. Alteration of the DIS affects RNA dimerization, packaging and reverse transcription, and reduces viral infectivity. The DIS loop initiates genome dimerization by forming a loop-loop complex and is further stabilized, putatively into an extended duplex form upon interaction with viral the NCp7 nucleocapsid protein. We have solved crystal structures of the DIS kissing-loop and extended duplex. Our structures revealed similarities with the bacterial 16S ribosomal RNA A-site, which is the target of aminoglycoside antibiotics. As a result, we have shown that aminoglycosides bind the HIV-1 DIS. Surprisingly, the affinity of these molecules for the DIS kissing-loop is higher than for their natural target in bacterial ribosomes. Their binding stabilizes strongly the loop-loop complex and prevents its conversion into the duplex form, even in presence of NCp7. We also solved numerous high-resolution X-ray structures of DIS kissing-loop complex and extended duplex bound to several aminoglycosides. However, identification of molecular driving forces important for the DIS/aminoglycoside binding could not be achieved by consideration of structural data alone, stressing the importance of collecting both thermodynamic and structural data for a complete understanding of the molecular recognition process.
Using ITC microcalorimetry, thermodynamics of aminoglycosides binding to the HIV-1 DIS duplex and kissing-loop forms was investigated over a range of conditions. Together with structural data provided by high-resolution crystal structures, we could establish the basis for the specificity of drug/RNA recognition and allow discriminating between specific binding sites and potential competing secondary sites. We could also show that drug binding to the DIS kissing-loop complex inhibits the NCp7-assisted conversion into the extended duplex form. By using all these data, we could rationally design an aminoglycoside conjugate that specifically binds the HIV-1 DIS RNA. Our results show the feasibility of targeting the HIV-1 DIS dimer before and after the NCp7-assisted RNA maturation with the same molecule.


Selected publications
- Ennifar E, Walter P, Ehresmann B, Ehresmann C, Dumas P (2001). Crystal structures of coaxially stacked kissing complexes of the HIV-1 RNA dimerization initiation site. Nat Struct Biol, 8(12):1064-8.
- Ennifar E, Paillart JC, Marquet R, Ehresmann B, Ehresmann C, Dumas P, Walter P (2003). HIV-1 RNA dimerization initiation site is structurally similar to the ribosomal A site and binds aminoglycoside antibiotics. J Biol Chem, 278(4):2723-30.
- Bernacchi S, Ennifar E, Tóth K, Walter P, Langowski J, Dumas P (2005). Mechanism of hairpin-duplex conversion for the HIV-1 dimerization initiation site. J Biol Chem, 280(48):40112-21.
- Ennifar E, Paillart JC, Bodlenner A, Walter P, Weibel JM, Aubertin AM, Pale P, Dumas P, Marquet R (2006). Targeting the dimerization initiation site of HIV-1 RNA with aminoglycosides : from crystal to cell. Nucleic Acids Res, 34(8):2328-39.
- Bernacchi S, Freisz S, Maechling C, Spiess B, Marquet R, Dumas P, Ennifar E (2007).Aminoglycoside binding to the HIV-1 RNA dimerization initiation site : thermodynamics and effect on the kissing-loop to duplex conversion. Nucleic Acids Res, 35(21):7128-39.
- Freisz S, Lang K, Micura R, Dumas P, Ennifar E (2008). Binding of aminoglycoside antibiotics to the duplex form of the HIV-1 genomic RNA dimerization initiation site. Angew Chem Int Ed Engl, 47(22):4110-3.
- Ennifar E, Aslam MW, Strasser P, Hoffmann G, Dumas P, van Delft FL (2013). Structure-guided discovery of a novel aminoglycoside conjugate targeting HIV-1 RNA viral genome. ACS Chem Biol, 8(11):2509-17.
 Intranet
Intranet Access
Access Contact
Contact
